Analysis from Weill Cornell Drugs reveals that astrocyte receptors influence cognitive features in a different way in women and men, suggesting a necessity for sex-specific approaches in creating remedies focusing on these mind cells.
Scientists at Weill Cornell Drugs have found the primary proof that receptors in astrocytes, mind cells that help and regulate neurons, can have contrasting results on cognitive perform in female and male preclinical fashions. This analysis highlights the position of astrocytes in contributing to gender-specific mind mechanisms.
Whereas many research have examined the behavioral results of astrocytic receptors, none of them have addressed whether or not organic intercourse performs a task and most have examined solely males. This research, printed on Could 24 in Cell Studies, challenges the long-standing assumption that astrocytic signaling has related cognitive results in each sexes.
“Our research reveals that beforehand reported cognitive results in males can’t be extrapolated to females,” mentioned Dr. Anna G. Orr, the Nan and Stephen Swid Assistant Professor of Frontotemporal Dementia Analysis and an assistant professor of neuroscience within the Feil Household Mind and Thoughts Analysis Institute and the Helen and Robert Appel Alzheimer’s Analysis Institute at Weill Cornell Drugs.
Modifications in astrocytic receptors are seen in varied neurological circumstances with identified intercourse variations, together with neurodegenerative issues, schizophrenia, stroke, and epilepsy. Nevertheless, the mechanisms selling intercourse variations stay poorly understood.
How do Male and Feminine Brains Differ?
Within the research, Dr. Samantha M. Meadows, first writer and former graduate scholar within the Orr lab, targeted on mGluR3, a predominant glutamate receptor in astrocytes and a top-altered gene in dementia. The crew used gene modifying and stimulation of engineered receptors in animal fashions to selectively manipulate astrocytes and study the results of mGluR3 and associated receptors on studying, reminiscence, and different cognitive and behavioral outcomes.
The researchers discovered that rising astrocytic mGluR3 ranges enhanced reminiscence in older females and lowering these ranges was ample to impair reminiscence in younger females, demonstrating that mGluR3 promotes reminiscence recall in females. Nevertheless, in males, lowering mGluR3 enhanced reminiscence, and rising the receptor had no results. “Apparently, the cognitive influence of those receptors is just not conserved amongst sexes,” Dr. Meadows mentioned.
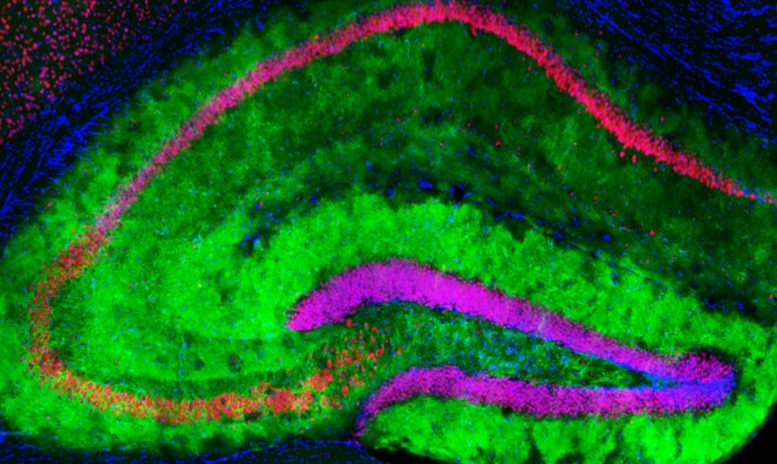
This picture of the mouse hippocampus, part of the mind concerned in studying and reminiscence, reveals mGluR3 receptors on astrocytes (inexperienced), neurons (purple), and cell nuclei (blue). Credit score: Orr Lab
To know if these divergent results had been distinctive to mGluR3 or mirrored a broader characteristic of astrocytic receptor signaling, Dr. Meadows labored with co-author Dr. Adam L. Orr, an assistant professor of analysis in neuroscience within the Mind and Thoughts Analysis Institute and the Appel Alzheimer’s Illness Analysis Institute, to selectively stimulate totally different astrocytic receptors whereas mice carried out duties involving studying and reminiscence.
To their shock, the crew discovered additional proof that receptor activation precipitated both reminiscence enhancement or impairment, relying on organic intercourse. “Regular mind perform appears to require a sex-specific steadiness in astrocytic signaling,” Dr. Adam Orr mentioned.
This research means that mGluR3 modulators being developed for treating issues reminiscent of schizophrenia and nervousness might have additional research to evaluate their influence on totally different sexes. “Therapeutics influencing astrocytic receptors could trigger sex-specific cognitive results partly as a result of divergent roles of astrocytes in women and men,” mentioned Dr. Anna Orr.
The lab is investigating what could trigger the differential results and if different mind features are additionally modified in a sex-specific method.
Reference: “Hippocampal astrocytes induce sex-dimorphic results on reminiscence” by Samantha M. Meadows, Fernando Palaguachi, Minwoo Wendy Jang, Avital Licht-Murava, Daniel Barnett, Until S. Zimmer, Constance Zhou, Samantha R. McDonough, Adam L. Orr and Anna G. Orr, 24 Could 2024, Cell Studies.
DOI: 10.1016/j.celrep.2024.114278